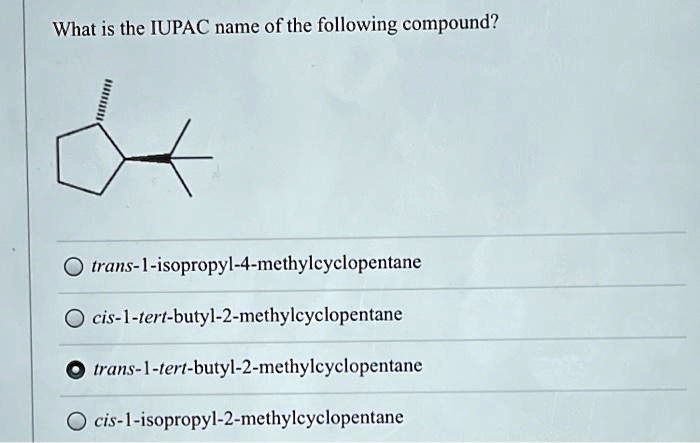
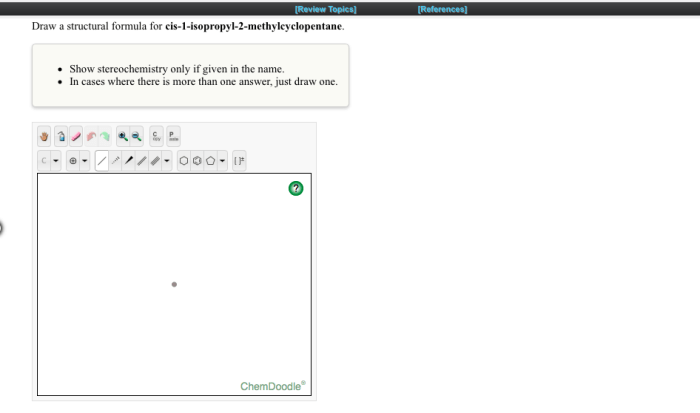
Cis 1 isopropyl 2 methylcyclopentane – Cis-1-isopropyl-2-methylcyclopentane, an organic compound with a captivating molecular structure, takes center stage in this discourse. Its physical and chemical properties, synthesis methods, reactivity, applications, and safety considerations will be meticulously examined, unveiling the multifaceted nature of this intriguing substance.
Properties
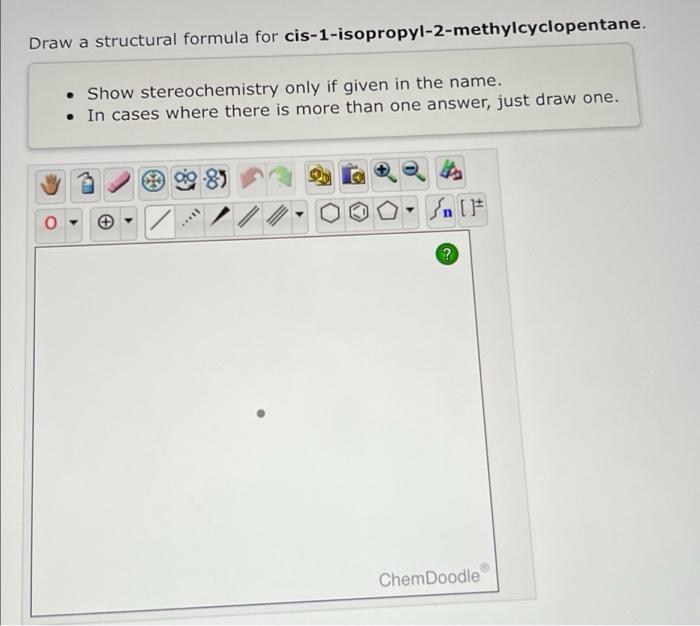
Cis-1-isopropyl-2-methylcyclopentane is a cycloalkane with the molecular formula C 8H 16. It is a colorless liquid with a mild odor. It is insoluble in water but soluble in organic solvents.
The molecular structure of cis-1-isopropyl-2-methylcyclopentane is a five-membered ring with two methyl groups and an isopropyl group attached to the ring. The two methyl groups are on the same side of the ring, and the isopropyl group is on the opposite side.
Physical Properties
- Molecular weight: 112.21 g/mol
- Density: 0.78 g/cm 3
- Boiling point: 156 °C
- Melting point: -93 °C
- Refractive index: 1.42
Chemical Properties, Cis 1 isopropyl 2 methylcyclopentane
- Cis-1-isopropyl-2-methylcyclopentane is a reactive hydrocarbon that can undergo a variety of reactions, including combustion, halogenation, and alkylation.
- It is a good solvent for nonpolar compounds.
- It is a weak acid and can react with strong bases to form salts.
Synthesis
Cis-1-isopropyl-2-methylcyclopentane can be synthesized through various methods, including:
Alkylation of Cyclopentene
In this method, cyclopentene undergoes alkylation with isopropyl bromide and methyl iodide in the presence of a Lewis acid catalyst, such as aluminum chloride or boron trifluoride. The reaction proceeds via an electrophilic addition mechanism, resulting in the formation of the desired product.
Hydroboration-Oxidation
This approach involves the hydroboration of 1-methylcyclopentene with diborane, followed by oxidation with hydrogen peroxide. The resulting organoborane intermediate undergoes rearrangement to form the cis-isomer of 1-isopropyl-2-methylcyclopentane.
Ring-Closing Metathesis
This method utilizes a ring-closing metathesis reaction between 5-hexen-1-ol and 3-buten-1-ol. The reaction is catalyzed by a Grubbs catalyst, leading to the formation of the cyclic product with the desired cis-configuration.
Reactivity: Cis 1 Isopropyl 2 Methylcyclopentane

Cis-1-isopropyl-2-methylcyclopentane is a reactive hydrocarbon due to the presence of both a double bond and an alkyl group. It undergoes a variety of reactions, including addition, substitution, and oxidation.
Addition Reactions
Cis-1-isopropyl-2-methylcyclopentane undergoes addition reactions with a variety of reagents, including hydrogen, halogens, and water. In these reactions, the double bond is broken and the new atoms or groups are added to the carbon atoms that were previously part of the double bond.
- Hydrogenation:Addition of hydrogen to cis-1-isopropyl-2-methylcyclopentane results in the formation of 1-isopropyl-2-methylcyclopentane.
- Halogenation:Addition of halogens (X2) to cis-1-isopropyl-2-methylcyclopentane results in the formation of 1-isopropyl-2-methyl-1,2-dihalocyclopentane.
- Hydration:Addition of water to cis-1-isopropyl-2-methylcyclopentane results in the formation of 1-isopropyl-2-methylcyclopentanol.
Substitution Reactions
Cis-1-isopropyl-2-methylcyclopentane also undergoes substitution reactions, in which one of the hydrogen atoms on the double bond is replaced by another atom or group. The most common type of substitution reaction is electrophilic addition, in which an electrophile (a species that is attracted to electrons) attacks the double bond.
- Electrophilic addition:Electrophilic addition of hydrogen halides (HX) to cis-1-isopropyl-2-methylcyclopentane results in the formation of 1-isopropyl-2-methyl-1-halocyclopentane.
- Electrophilic addition:Electrophilic addition of sulfuric acid (H2SO4) to cis-1-isopropyl-2-methylcyclopentane results in the formation of 1-isopropyl-2-methylcyclopentyl hydrogen sulfate.
Oxidation Reactions
Cis-1-isopropyl-2-methylcyclopentane undergoes oxidation reactions, in which the double bond is broken and the carbon atoms that were previously part of the double bond are oxidized to form carbonyl groups (C=O). The most common type of oxidation reaction is permanganate oxidation, in which potassium permanganate (KMnO4) is used to oxidize the double bond.
- Permanganate oxidation:Permanganate oxidation of cis-1-isopropyl-2-methylcyclopentane results in the formation of 1-isopropyl-2-methylcyclopentanone.
Applications
Cis 1-isopropyl-2-methylcyclopentane is employed in various industrial and research applications. Its unique properties make it a valuable intermediate and solvent in several processes.
As an intermediate, it plays a crucial role in the synthesis of more complex organic compounds, including pharmaceuticals, fragrances, and flavors. Its reactivity allows for efficient functionalization and modification, enabling the production of a wide range of target molecules.
As a Solvent
Cis 1-isopropyl-2-methylcyclopentane exhibits excellent solvent properties due to its non-polar nature and ability to dissolve a variety of organic compounds. It is commonly used as a solvent in paints, coatings, and adhesives, providing good film formation and adhesion characteristics.
Safety Considerations
Cis 1 isopropyl 2 methylcyclopentane is a flammable liquid with a flash point of20 °C. It is also harmful if swallowed, inhaled, or absorbed through the skin. Contact with the liquid can cause skin and eye irritation.
Handling
When handling cis 1 isopropyl 2 methylcyclopentane, it is important to take the following precautions:
- Wear appropriate personal protective equipment, including gloves, eye protection, and a respirator.
- Use in a well-ventilated area.
- Avoid contact with skin and eyes.
- Do not breathe in vapors.
- Keep away from heat, sparks, and open flames.
Storage
Cis 1 isopropyl 2 methylcyclopentane should be stored in a cool, dry place away from heat, sparks, and open flames. It should be stored in a tightly closed container to prevent evaporation.
Disposal
Cis 1 isopropyl 2 methylcyclopentane should be disposed of in accordance with local, state, and federal regulations. It should not be disposed of in the trash or down the drain.
Question & Answer Hub
What are the physical properties of cis-1-isopropyl-2-methylcyclopentane?
It is a colorless liquid with a boiling point of 156-157 °C and a density of 0.79 g/cm³.
How can cis-1-isopropyl-2-methylcyclopentane be synthesized?
Various methods exist, including the Diels-Alder reaction between isoprene and methyl acrylate, followed by hydrogenation.
What are the applications of cis-1-isopropyl-2-methylcyclopentane?
It is used as an intermediate in the synthesis of fragrances, flavors, and pharmaceuticals.