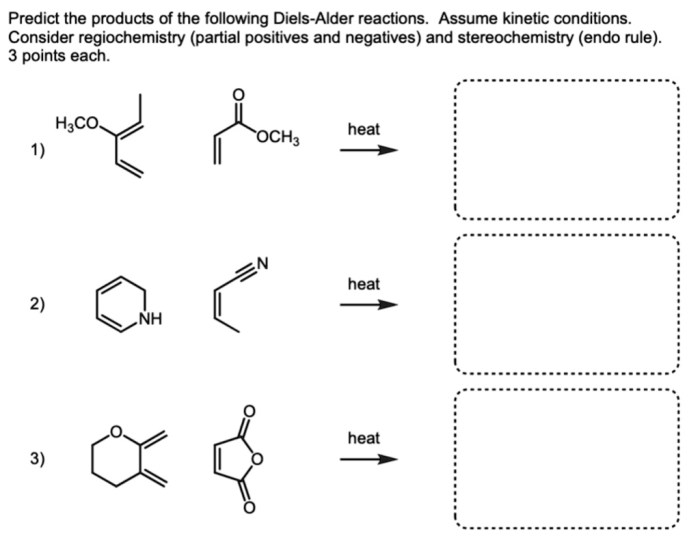
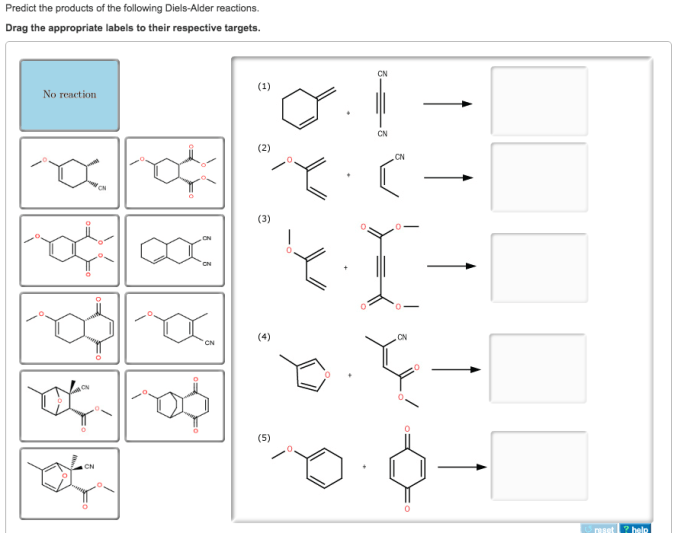
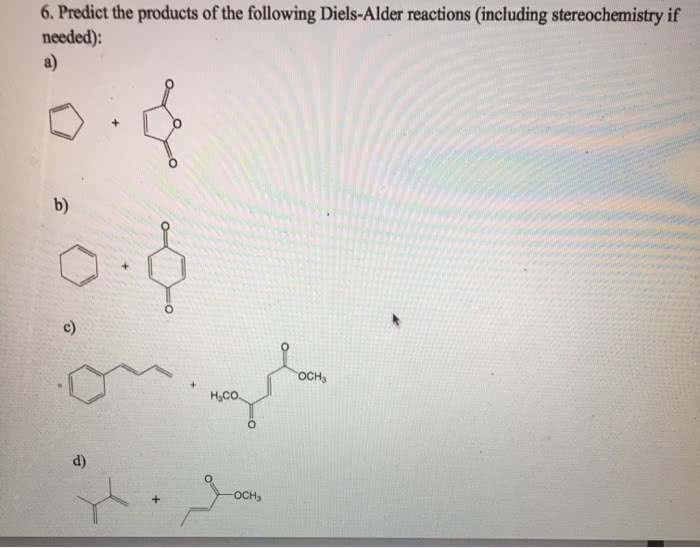
Predict the products of the following diels-alder reactions. – Diels-Alder reactions are a cornerstone of organic chemistry, enabling the construction of complex molecules with remarkable efficiency. This guide delves into the intricacies of predicting the products of these reactions, empowering chemists with the knowledge to harness their synthetic potential.
Predicting the products of Diels-Alder reactions is crucial for planning synthetic strategies and understanding reaction mechanisms. Factors such as regio- and stereoselectivity play a significant role in determining the outcome, and this guide provides a comprehensive overview of the methods used to predict these aspects.
Predicting Products of Diels-Alder Reactions: Predict The Products Of The Following Diels-alder Reactions.
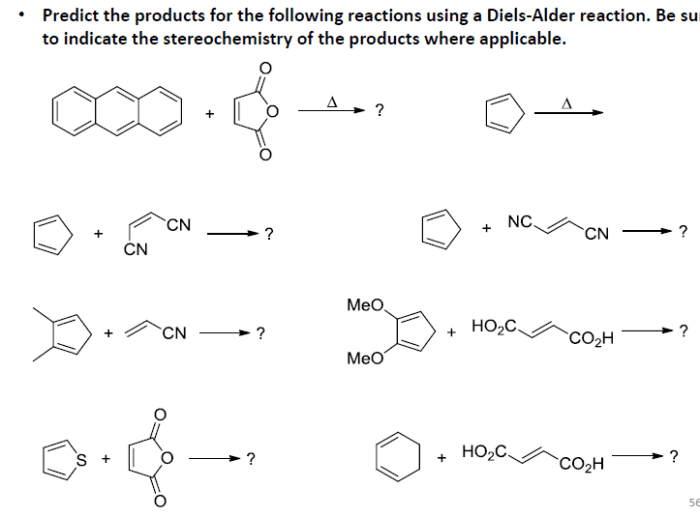
Diels-Alder reactions are one of the most powerful tools in organic chemistry for constructing cyclic compounds. They involve the cycloaddition of a conjugated diene with a dienophile, resulting in the formation of a cyclohexene ring.
The mechanism of Diels-Alder reactions is concerted and involves the formation of a new σ-bond between the diene and the dienophile and the breaking of two π-bonds in the diene. The stereochemistry of the reaction is determined by the relative orientations of the diene and the dienophile.
When the diene and the dienophile are in an endo orientation, the reaction proceeds through a transition state that is more compact and has less steric hindrance, resulting in the formation of an endo product. When the diene and the dienophile are in an exo orientation, the reaction proceeds through a transition state that is less compact and has more steric hindrance, resulting in the formation of an exo product.
Predicting the Products of Diels-Alder Reactions
Predicting the products of Diels-Alder reactions is important for planning synthetic strategies and for understanding the mechanisms of these reactions. The regioselectivity of a Diels-Alder reaction is determined by the relative reactivities of the different double bonds in the diene and the dienophile.
The stereoselectivity of a Diels-Alder reaction is determined by the relative orientations of the diene and the dienophile.
There are a number of factors that influence the regio- and stereoselectivity of Diels-Alder reactions. These factors include the electronic properties of the diene and the dienophile, the steric effects of the substituents on the diene and the dienophile, and the solvent effects.
Methods for Predicting Products
There are a number of methods for predicting the products of Diels-Alder reactions. These methods include the Woodward-Hoffmann rules, frontier molecular orbital (FMO) theory, and computational methods.
The Woodward-Hoffmann rules are a set of rules that can be used to predict the regioselectivity of Diels-Alder reactions. The rules are based on the conservation of orbital symmetry and can be used to predict the relative reactivities of different double bonds in the diene and the dienophile.
FMO theory is a method that can be used to predict the stereoselectivity of Diels-Alder reactions. FMO theory is based on the interactions between the frontier molecular orbitals of the diene and the dienophile. The frontier molecular orbitals are the highest occupied molecular orbital (HOMO) of the diene and the lowest unoccupied molecular orbital (LUMO) of the dienophile.
Computational methods are a powerful tool for predicting the products of Diels-Alder reactions. Computational methods can be used to calculate the energies of the different transition states for a given Diels-Alder reaction and to predict the relative reactivities of these transition states.
Examples, Predict the products of the following diels-alder reactions.
The following are some examples of Diels-Alder reactions with different dienes and dienophiles:
- The reaction of 1,3-butadiene with maleic anhydride gives a mixture of endo and exo products.
- The reaction of 1,3-butadiene with acrylic acid gives a mixture of endo and exo products.
- The reaction of 1,3-butadiene with methyl vinyl ketone gives a mixture of endo and exo products.
The regio- and stereoselectivity of these reactions can be predicted using the Woodward-Hoffmann rules, FMO theory, and computational methods.
Applications
Diels-Alder reactions are used in a wide variety of organic synthesis applications. These applications include the synthesis of natural products, pharmaceuticals, and polymers.
One of the most important applications of Diels-Alder reactions is the synthesis of natural products. Diels-Alder reactions have been used to synthesize a wide variety of natural products, including steroids, alkaloids, and terpenes.
Diels-Alder reactions are also used in the synthesis of pharmaceuticals. Diels-Alder reactions have been used to synthesize a wide variety of pharmaceuticals, including antibiotics, anti-cancer drugs, and anti-inflammatory drugs.
Diels-Alder reactions are also used in the synthesis of polymers. Diels-Alder reactions have been used to synthesize a wide variety of polymers, including polyesters, polyamides, and polyurethanes.
Detailed FAQs
What are the key factors that influence the regioselectivity of Diels-Alder reactions?
The regioselectivity of Diels-Alder reactions is primarily governed by the electronic properties of the diene and dienophile, as well as steric effects.
How can frontier molecular orbital theory be used to predict the stereoselectivity of Diels-Alder reactions?
Frontier molecular orbital theory provides a qualitative understanding of the stereochemical outcome of Diels-Alder reactions by analyzing the interactions between the highest occupied molecular orbital (HOMO) of the diene and the lowest unoccupied molecular orbital (LUMO) of the dienophile.