Isopropyl alcohol heat of vaporization, a crucial property that governs its diverse applications, unveils a fascinating realm of intermolecular interactions and practical implications. This exploration delves into the intricate relationship between heat of vaporization and the unique characteristics of isopropyl alcohol, shedding light on its versatility as a solvent, disinfectant, and more.
As we delve into the intricacies of isopropyl alcohol’s heat of vaporization, we uncover its role in shaping its physical and chemical properties, influencing its behavior in various applications. Join us on this scientific expedition as we unravel the secrets behind this remarkable substance.
Properties of Isopropyl Alcohol
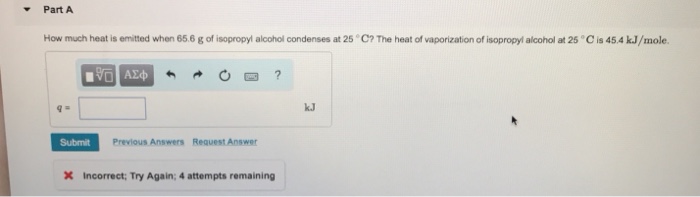
Isopropyl alcohol, also known as 2-propanol, is a colorless, flammable liquid with a characteristic odor. It is a versatile solvent that is widely used in various industrial and household applications. The physical and chemical properties of isopropyl alcohol are summarized in the following table:| Property | Value ||—|—|| Molecular formula | C 3H 8O || Molecular weight | 60.10 g/mol || Density | 0.786 g/mL || Melting point |
89.5 °C |
| Boiling point | 82.6 °C || Heat of vaporization | 42.8 kJ/mol || Flash point | 12 °C || Autoignition temperature | 455 °C |The heat of vaporization of a liquid is the amount of energy required to convert one mole of the liquid to a gas at its boiling point.
The heat of vaporization of isopropyl alcohol is 42.8 kJ/mol, which is relatively high compared to other alcohols. This high heat of vaporization indicates that strong intermolecular forces exist between isopropyl alcohol molecules. These intermolecular forces include hydrogen bonding, dipole-dipole interactions, and van der Waals forces.
Applications of Isopropyl Alcohol

Isopropyl alcohol is a versatile solvent that is used in a wide variety of applications, including:
- As a solvent:Isopropyl alcohol is a good solvent for many organic compounds, including oils, greases, and waxes. It is also used as a solvent in paints, varnishes, and cleaning solutions.
- As a disinfectant:Isopropyl alcohol is a powerful disinfectant that is effective against a wide range of bacteria and viruses. It is commonly used to disinfect surfaces, medical instruments, and skin.
- As a fuel:Isopropyl alcohol is a clean-burning fuel that can be used in internal combustion engines. It is also used as a fuel in camping stoves and other portable heating devices.
The heat of vaporization of isopropyl alcohol plays an important role in its use as a solvent and disinfectant. The high heat of vaporization of isopropyl alcohol makes it a good solvent for non-polar compounds, such as oils and greases.
The high heat of vaporization also makes isopropyl alcohol a good disinfectant, as it kills bacteria and viruses by denaturing their proteins.
Measurement of Heat of Vaporization

The heat of vaporization of isopropyl alcohol can be measured using a variety of experimental methods, including:
- Calorimetry:In this method, a known mass of isopropyl alcohol is heated in a calorimeter until it boils. The heat of vaporization is calculated from the amount of heat absorbed by the calorimeter.
- Gas chromatography:In this method, a known volume of isopropyl alcohol is injected into a gas chromatograph. The heat of vaporization is calculated from the retention time of the isopropyl alcohol peak.
The accuracy of these measurements can be affected by a number of factors, including the purity of the isopropyl alcohol, the temperature of the experiment, and the accuracy of the measuring equipment.
Comparison with Other Alcohols: Isopropyl Alcohol Heat Of Vaporization
The heat of vaporization of isopropyl alcohol is higher than that of other alcohols with similar molecular weights. For example, the heat of vaporization of methanol is 35.2 kJ/mol, and the heat of vaporization of ethanol is 38.6 kJ/mol. This difference in heat of vaporization is due to the increased branching of the isopropyl alcohol molecule.
The branching of the isopropyl alcohol molecule results in weaker intermolecular forces, which leads to a higher heat of vaporization.The higher heat of vaporization of isopropyl alcohol has implications for its applications. For example, isopropyl alcohol is a more effective disinfectant than methanol or ethanol, as it has a higher boiling point and is more resistant to evaporation.
Safety Considerations
Isopropyl alcohol is a flammable liquid that can cause skin irritation and respiratory problems. It is important to take the following safety precautions when working with isopropyl alcohol:
- Use isopropyl alcohol in a well-ventilated area.
- Avoid contact with skin and eyes.
- Do not ingest isopropyl alcohol.
- Store isopropyl alcohol in a cool, dry place away from heat and open flames.
The high heat of vaporization of isopropyl alcohol can pose a potential hazard, as it can lead to the formation of flammable vapors. It is important to avoid using isopropyl alcohol near open flames or other sources of ignition.
Questions and Answers
What is the significance of heat of vaporization in understanding isopropyl alcohol?
Heat of vaporization provides insights into the intermolecular forces within isopropyl alcohol, revealing the strength of the interactions between its molecules.
How does heat of vaporization affect the efficiency of isopropyl alcohol as a solvent?
Heat of vaporization influences the ability of isopropyl alcohol to dissolve other substances, impacting its effectiveness as a solvent in various applications.
What safety precautions should be taken when working with isopropyl alcohol due to its high heat of vaporization?
Proper ventilation and handling techniques are crucial to minimize exposure to isopropyl alcohol vapors, which can pose potential health hazards.